These Robots Are Self-Replicating. Or Are They?
The headline is dystopian clickbait at its finest. World’s first living robots can now reproduce. To the uninitiated, this announcement conjures visions of Westworld, Blade Runner, and the Borg. It gets the intended response. Hundreds of speed readers smash the quote-Tweet in chorus, deadpanning apocalyptic takes and Battlestar Galactica GIFs. The reactions write themselves: We have living robots, and now they’re breeding? What could possibly go wrong? Of course, these posts riff on the headline alone—a headline that sells the sizzle of a news article serving only the meatiest bits of a sci-comms press release, which, in turn, attempts to make a scientific paper digestible to the general public. Back in the lab, there’s a team of scientists trying to draw attention—and funding—to work that just might shift entire biological paradigms. Just not in a Battlestar Galactica kind of way.
The “living robots” in question are far from menacing, multiplying androids. They’re the size of a quartered poppy seed, for one, and they live only ten days. In fact, they’re nothing more threatening than a cluster of cells culled from a frog embryo, sculpted into a shape ordained by an evolutionary algorithm, and set free to perform minute errands in a Petri dish. Developed by an interdisciplinary team at Tufts, Harvard, and the University of Vermont, these tiny experimental creatures, known as Xenobots, may someday transport targeted medicine within the body or metabolize plastic in the ocean.
For now, however, Xenobots defy attempts at basic categorization with amphibian slipperiness. They’re alive, but they were never born; they’re manufactured, but come pre-loaded with biological wetware, which helps them solve problems, heal themselves and, as of late last year, replicate. Every new revelation about these unusually capable frog cells opens up a new can of worms—or perhaps tadpoles—re-igniting a niche but intense debate about biology, technology, and the responsibilities of scientific communication. How alive are Xenobots, really? Is it accurate to describe them as robots? Are they really replicating? And how does one separate a microscopic breakthrough from all the big stories told about it?
Natural Limits
Xenobots certainly look alive. Propelled by tiny cilia, they swirl around their Petri dish like “Roombas in ballistic trajectories,” as a member of the Xenobot team once described it to me. When they encounter loose embryonic frog cells, that swirling begins to look a lot like herding; the mobile Xenobots scoop loose cells into piles, which naturally bind together, forming new Xenobots that continue this same process of swirling, herding, and making more Xenobots. “They started corralling inert particles. I saw them making heaps,” the lead biologist on the project, Michael Levin of Tufts, recalled in a recent interview. “They’re basically making this Xenobot in front of our eyes.” Sidestepping the question of biological reproduction, the research team calls this process “kinematic self-replication.”
Basic Xenobots can spawn three generations of offspring in this way; Xenobots that have been surgically sculpted into a semi-torus shape (almost exclusively referred to as “Pac Man-shaped”) are able to replicate across five generations. But they will not overrun the Petri dish anytime soon. Xenobot replication is a delicate and tenuous operation: it only works when a feedstock of fresh cells is being pumped into the environment by an experimenter, and it’s far from exact. The function of first-generation Xenobots is determined by their form, but their offspring are more haphazard—just cells bumping around and cohering into roundish clumps. On their own, all Xenobots wither and die within two weeks. Fortunately, they’re biodegradable.
Members of the team believe there may be ways to transcend these natural limitations. Asked whether he thinks Xenobots could eventually replicate in perpetuity, Levin is happy to answer with a clear “we don’t know,” but follows up with informed speculation. Because of their short lifespans, he explains, Xenobots are only able to “replicate” a few times. If his team were to add nutrients to the Xenobots’ environment, however, they might live longer. “If we were to feed them, which we do know how to do,” Levin claimed recently, “maybe they would have the energy to make piles in perpetuity.”
Size is another consideration. Bigger Xenobots might have a commensurately larger impact on the world, but as it stands today, a Xenobot has an upper size limit of about one millimeter. Any larger and its cilia would be too weak to move its weight; its center, too, would be starved of oxygen. These natural barriers, Levin proposes, could be breached by equipping the Xenobot with a Sierpiński gasket, a kind of breathing scaffolding often used in cellular agriculture.
For now, the team emphasizes that they’re seeing how far they can get with minimal human intervention, to establish where biology goes on its own accord. In the meantime, journalists, science communicators, and potential funders demand educated predictions, and Levin is happy to spin implications and applications alike. A big-picture scientist made for the big stage, he’s expert at situating Xenobots in the widest evolutionary context, connecting the microscopic present to a future vast with possibilities, from environmental remediation to regenerative medicine. This balancing act has drummed up enormous excitement for the tiny creatures—and opened up the project to fundamental scrutiny.
Fuzzy Definitions
Late last year, Ars Technica science editor John Timmer praised Xenobot research as “generally interesting,” while criticizing what he saw as the team’s penchant for overstatement. Xenobots couldn’t fairly be described as replicating, for example, because they weren’t creating true copies of themselves. Really, the team had just “optimized a way of getting mobile clusters of cells to organize other cells into smaller clusters.” Further, he argued that the team were partially responsible for the exaggerated headlines about their work because of their own fast and loose use of terminology. But when Timmer accuses the team of being “inherently lazy,” one starts to get the impression that charismatic projects like Xenobots can easily become scapegoats for the rhetorical gimmicks and PR-driven hype increasingly necessary to secure funding for scientific research.
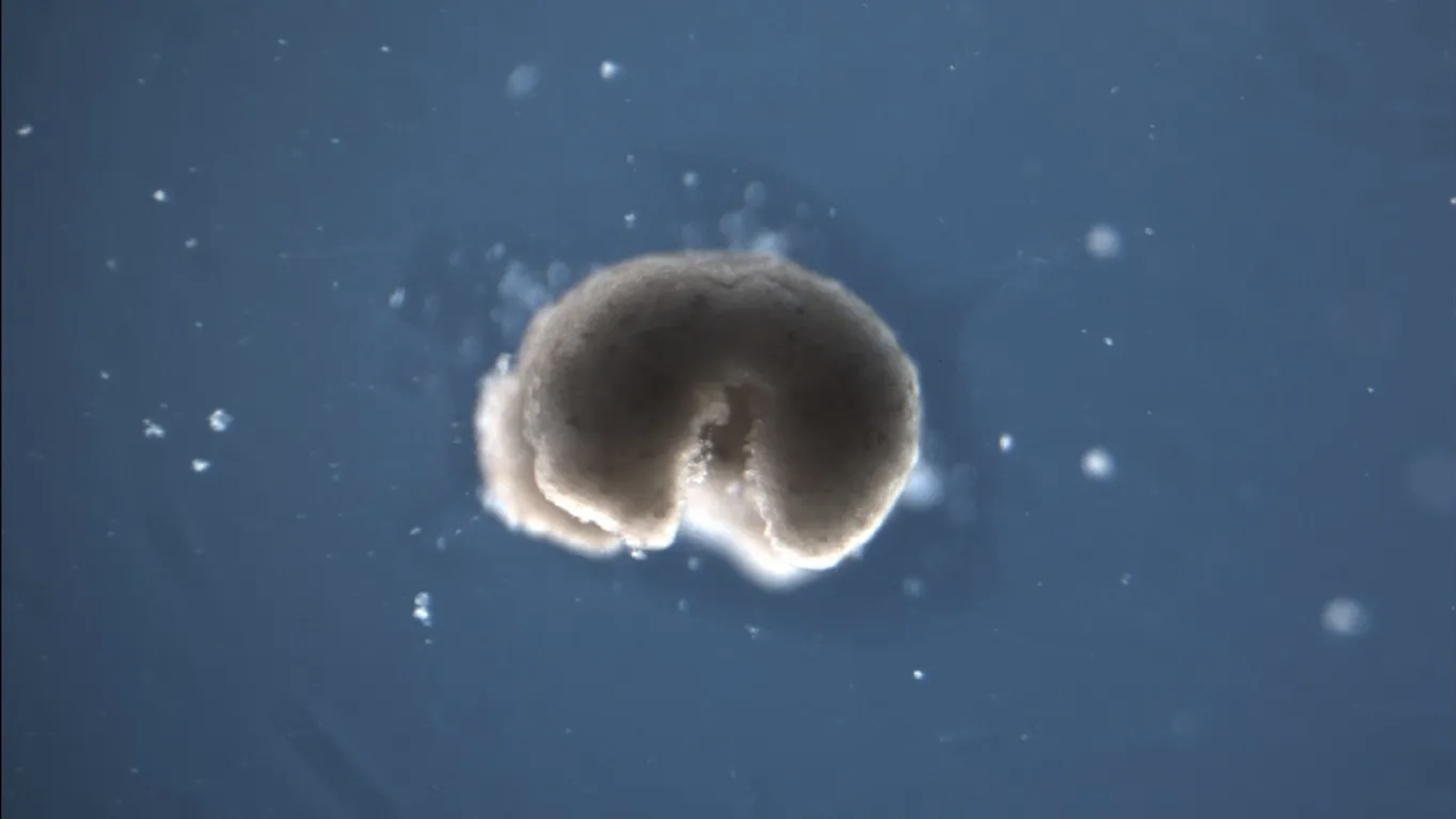
On social media, the most common complaint lodged against the project is that Xenobots are actually nothing new. In developmental biology, developing frog cells excised from an embryo and left to cohere into a ball of skin tissue is known as an “animal cap.” Animal cap assays are common stuff, and their unusual behaviors have been studied for decades, leading some more vocal developmental biologists to state that Xenobots are, at best, animal caps rebranded for the sake of hype. But those who have read the Xenobot papers closely recognize that animal caps are only part of the pipeline, which—guided by modeling, evolutionary computing, and simulation—has produced a far more diverse array of biological robots.
Some Xenobots contain muscle-derived tissues, for example. Douglas Blackiston, the developmental biologist on the team, has painstakingly sculpted and carved a variety of different AI-generated Xenobot forms, optimized for different tasks and abilities. The online ire seems to be more about language than science; calling an animal cap a robot, to some biologists, seems disingenuous, and signals the unwelcome incursion of a certain engineering mentality to ideas already being explored in the life sciences. “Roboticists and developmental biologists don't talk the same language,” says Blackiston, “and the public doesn't talk the same language,” either.
Blackiston argues that many terms central to both biology and robotics lack common operational definitions—and often have contradictory interpretations. “If you get ten biologists into a room and ask, ‘what's an organism?’” he explains, “it's like a knife fight in a phone booth.” The word “robot” is equally unclear. Is a robot defined by its materiality—what it is made of—or is a robot what a robot does? Likely motivated by these questions, the Xenobots team recently shared a fascinating preprint addressing their semantic and conceptual difficulties. In the manuscript, each member of the team shares their own divergent perspective on the emerging question of bio-robotic nomenclature. In his section, roboticist Sam Kriegman acknowledges that “parts of animals are not robots.” But once they have been artificially combined and shaped to “render desired behaviors, they are no longer merely animal parts, they become artifacts: artificial yet fully biological robots.”
By this definition, incidentally, Frankenstein is a robot.
Model Cells
Perhaps Xenobots owe some of their special charge to the host organism from which they emerge—a peculiar clawed frog, Xenopus laevis, known across sub-Saharan Africa for its aphrodisiac properties. Nearly a century ago, these frogs took an unexpected scientific commission, becoming a model organism in biology. Their circuitous path to the wet lab began with the British zoologist Lancelot Hogben, a passionate leftist and one of the only scientists of his generation to renounce the eugenics movement. In the late 1920s, Hogben was the chair of zoology at the University of Cape Town, where Xenopus was abundant in nearby ponds and rivers. Easy to trap and compellingly chameleonic, the local frog became a frequent subject in Hogben’s endocrinology lab.
In 1930, outraged by South Africa’s racism, Hogben returned to London—with a colony of frogs in tow. Back home, Hogben found that when injected with a pregnant woman’s urine, the female Xenopus frog ovulated within hours, releasing a grip of slimy black-and-white eggs. The “Hogben test” became the standard international pregnancy test for 15 years. As a consequence, colonies of Xenopus frogs were established around the world.
From pond to Petri dish, the Xenopus frog has been nudged along by unexpected discoveries and disturbed by political, ethical, and social forces. Had Lancelot Hogben not been so principled, Xenopus laevis would likely never have become a model organism in biology. In turn, the Hogben test normalized pregnancy testing, giving women more control over their reproductive choices. New life is not something to bring lightly into this world—not for women, and not for the creators of living robots, either.
And so Xenobots are just the latest chapter in the frogs’ entanglement with us. From sparking the lust to create new life, to identifying life’s earliest stirrings, and finally to providing the building blocks of a new form of life—one that can now, in a limited sense, replicate itself—Xenopus laevis has been a mascot for multiplicity, a metaphor for life’s irrepressible drive towards abundance and regeneration. Life resists the binaries of dichotomous thinking and the constraints of academic disciplines. It transcends and muddles boundaries, slippery as a frog. As confounding—and controversial—as Xenobots are, their very existence reveals the inadequacy of our current language for describing biology and technology alike.
Cognition. Intelligence. Reproduction. Organism. When it comes to Xenobots, even the most basic terms are debatable. The Xenobots team itself seems to be of at least two minds about how to think and talk about it all. Not long after I interviewed Kriegman and Blackiston for this piece, Blackiston sent me an email urging me not to use any “cognitive terms” to describe Xenobot behavior. Words like “think,” “goal,” and “intelligence,” he explained, are controversial in biology circles, and although Xenobots might someday be engineered to demonstrate complex programmable behaviors, those wouldn’t necessarily signal intelligence. “We have difficulty describing cognition even in the contexts of humans, so it's difficult to apply fuzzy terminology down the evolutionary tree,” he wrote. Not all of his colleagues share this caution, however, and the question of intelligence haunts the Xenobot project.
Nothing in any animal’s genome suggests that one of its cells might be able to organize with other cells into complex structures outside of its original body. But this is precisely what these frog cells do, when given the chance. Levin routinely argues that cells are “competent agents,” employing a bioelectric language to communicate with one another to form complex structures. Seeking to parse this language, his lab once scrambled the facial features of embryonic tadpoles; by the time the "Picasso Tadpoles” emerged as frogs, their mouths, noses and eyes had settled back into place, indicating that morphology—the coming together of the body—is guided not only by the genome, but also by bioelectric cues.
Levin sees Xenobots as an opportunity to discover what life gets up to when liberated from its evolutionary boundaries. When it is part of a multicellular organism, a cell has a job—to make skin, for example, with hairlike cilia to keep bacteria at bay. But if that same cell is placed in an entirely novel context and left to “reimagine its multicellularity,” as Levin puts it, what does it do? How can it organize with other cells? In a recent interview, he mused, “What did evolution actually learn when making the frog genome? It wasn’t just to make a good frog.…What I think is clear is that evolution doesn’t produce specific solutions to specific environmental problems. It produces problem-solving machines.” Of course, problem-solving machines are precisely what the roboticists on the team are interested in building.
Observing how single cells organize with one another and solve problems could inform future approaches to both Artificial Intelligence and robotics. Dr. Joshua Bongard, who runs the robotics and computer science side of the Xenobots effort at the University of Vermont, has frequently underlined the potential fruitfulness of a bottom-up approach to understanding cognition. Rather than fine-tuning the synaptic connections of artificial neural networks, he suggests, AI researchers might learn a great deal from studying how cells solve problems. “Intelligence is not something stored somewhere in the brain,” he explained during a recent webinar. “Whatever is going on is a much more complex dance between body, brain, environment, and other sentient beings.” Xenobots grew out of a belief that biology—all the way down to the humble cell—is collective, adaptive, and has much to teach us yet. Or, as the team put it in their most recent paper: “life harbors surprising behaviors just below the surface, waiting to be uncovered.” ♦
Subscribe to Broadcast